Sutent 50mg
- Sutent 50mg is an antineoplastic drug which prevents the growth and spread of cancer cells in the body.
- Sutent 50mg belongs to targeted therapy and is a receptor protein-tyrosine kinase inhibitor. It prohibits the actions of vascular endothelial growth factor (VEGF) and is an angiogenesis inhibitor.
- Sutent 50mg is a prescription drugs which is used under the guidance of medical practioners
INDICATION
- Sutent 50mg is used for the treatment in patients having Gastrointestinal stromal tumor
- Sutent 50mg is used for the treatment in patients having Advanced pancreatic neuroendocrine tumor
- Sutent 50mg is used for the treatment in patients having Advanced renal cell cancer
DOSAGE
The recommended dosage for following condition as follows :
- Dose after disease progression on or intolerance to imatinib mesylate
- The usual dose for patients with Gastrointestinal Stromal Tumor is 50 mg PO qDay for 4 weeks, followed by 2 weeks drug-free, repeat cycle
- The usual dose for patients with Renal Cell Carcinoma is 50 mg PO qDay for 4 weeks, followed by 2 weeks drug-free, repeat cycle
Adjuvant treatment of RCC :
- Used for the adjuvant therapy of adult patients at high risk of recurrent RCC following nephrectomy
- The recommended dose is 50 mg PO qDay for 4 weeks, followed by 2 weeks drug-free, repeat cycle for a maximum of nine 6-week cycles totally
- The usual dose for patients with Pancreatic Neuroendocrine Tumor is 37.5 mg PO qDay continuously without a scheduled off-treatment period
MECHANISM
- Sunitinib belong to type of small molecule which prohibits multiple RTKs, some of which are involved in tumor growth, pathologic angiogenesis, and metastatic progression of cancer. Sunitinib was judge for its inhibitory activity against a variety of kinases (>80 kinases) and was identified as an inhibitor of platelet-derived growth factor receptors (PDGFRa and PDGFRb), vascular endothelial growth factor receptors (VEGFR1, VEGFR2 and VEGFR3), stem cell factor receptor (KIT), Fms-like tyrosine kinase-3 (FLT3), colony stimulating factor receptor Type 1 (CSF-1R), and the glial cell-line derived neurotrophic factor receptor (RET)
ADME
- High plasma concentration of sunitinib is between 6 and 12 hours and the bioavailability of Sunitinib has no effect on food.
- Human plasma protein binding was 95 % and 90 % respectively.
- Primarily metabolized by cytochrome P450 enzyme, CYP3A4
- The drug Sunitinib is eliminated primarily through feces 61% and renal excretion is 16% of administrated dose. Sunitinib half-life is 40 – 60 hours
- Primary active metabolite half-life was 80 – 110 hours
PRECAUTION
- Adrenal haemorrhage resulted in animal studies; follow the adrenal function in case of stress such as surgery, trauma or severe infection
- Control urine protein; interfere with treatment for 24-hr urine protein ≥3 g; stop for repeat episodes of protein ≥3 g despite dose reductions or nephrotic syndrome
- Sutent 50mg treatment will causes severe hepatotoxicity involving liver failure; check liver function tests before starting treatment.
- Haemorrhagic events involving tumor-related haemorrhage resulted; test the serial complete blood counts and physical examinations
- Hypothyroidism resulted; follow the baseline thyroid function in patients with hypothyroidism or hyperthyroidism and treat per standard medical practice prior to initiating therapy
DRUG INTERACTION
- Sutent 50mg combination with CYP3A4 inhibitors (ketoconazole0 will increase the sunitinib plasma concentrations.
- Sutent 50mg combination with CYP3A4 inducers (rifampin) will decrease Sunitinib plasma concentration
CONTRAINDICATION
- Patients are contraindicated with Sutent 50mg due to hypersensitivity and renal impairment.
MISSED DOSE
- If dose is missed the have the dose immediately before next dose timing reaches or skip the missed dose and continue the regular schedule.
- Consult doctors regarding missed dose.
STORAGE
- Stored at controlled temperature at 25℃
- Keep away from children’s resistance
- Dispense only in its original carton
SIDE EFFECTS
Common side effects :
- Signs of allergic reactions: hives, breathing difficulty, sore throat, skin pain, red or purple skin rash Liver problem: upper stomach pain, itching, tried feeling, decreased appetite, dark urine, jaundice.
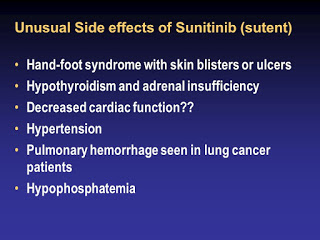
Serious side effects :
- Swelling in ankles or feet
- Fast heart beats
- Sudden dizziness
- Shortness of breath
- Unusual bruising
- Signs of tumor cell breakdown – muscle cramps, tiredness, decreased urination, slow heart rate.
- Thyroid problem – severe tiredness, depression, rapid heart rate, , feeling nervous, sweating, nausea, vomiting, diarrhea, loss of hair, weight changes, irregular menstrual periods.
CONTACT DETAILS
Phone : 9987711567
Email : applepharmaceutical@gmail.com
Email : info@myapplepharma.com
Website :