Lenvatinib 10mg capsule
- Lenvima 10mg is also known as tyrosine kinase inhibitor.
- Lenvima 10mg targets something to the cancer cells.
- Lenvima 10mg capsule is well absorbed. In which Food does not affect the range of absorption but lower the rate.
- Lenvima 10mg which is a prescription drug used under proper guidance of medical oncologist.
INDICATION
- Indicated For the treatment of thyroid cancer which cannot be treated with radioactive iodine.
- Indicated By combined with everolimus to treat the advanced renal carcinoma in which other drugs failed to be effective.
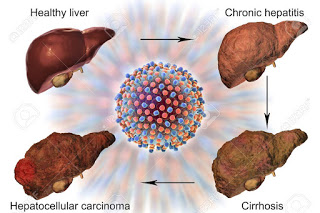
- Indicated For the treatment of hepatocellular primary liver cancer
DOSAGE
- The recommended dose of differentiated thyroid cancer is Lenvatinib 24 mg, it administers by two 10mg and one 4 mg capsules taken orally, once in a day along with or without food.
- The recommended dose of renal cell carcinomas is Lenvatinib 18 mg, given as one 10mg capsule and two 4 mg capsules administrated in combination with 5mg everolimus orally taken once in a day with or without food.
- The recommended dose of first-line treatment of unresectable hepatocellular carcinoma is based on actual body weight
- If < 60 kg then 8mg PO qDay
- If ≥ 60 kg then 12mg PO qDay
Administration :
- Lenvima 10mg capsule must take at same time each day
- Follow the drug till disease progression.
- Lenvima 10mg capsule must swallowed whole.
- In a small glass of liquid dissolved the capsules
- In 1 tablespoon apple juice or water and embed the capsules into the liquid without breaking or crushing them.
- Dip the capsules in the liquid for at least 10 minutes.
- The liquid is agitated for at least 3 minutes. Drink the mixture.
- Add 1 tablespoon of water or apple juice to the glass after drinking the mixture
- Mix up the contents a few times and swallow the additional liquid.
OVERDOSAGE:
- Lenvima 10mg has no individual antitoxin for overdose. Since high plasma protein binding. Lenvima 10mg capsule is not expected to be dialyzable. Take the patients to emergency medical department or call poison control helpline.
MECHANISM
Lenvima 10mg called as targeted therapy. In which it aims something particular to the cancer cells, hence reduces side effects caused by repairing to the healthy cells. Lenvima 10mg mechanism by inhibiting two processes which allow cancer cells to develop
- Prevention of a protein that promotes cell division
- Stopping the VEGF receptor, which is necessary for angiogenesis, or the growth of a blood supply to the tumor. This discard the tumor’s source of nutrients.
ADME
- Maximum plasma concentration is 1 to 4 hours post dose.
- Effect with food is low to the extent of absorption.
- Human plasma proteins bounding about 98% to 99%.
- Metabolized in CYP3A and aldehyde oxidase. Lenvatinib metabolic pathway is identified as enzymatic and non-enzymatic processes.
- The drug excreted via 64% feces and 25% urine,
- The terminal half-life of Lenvatinib is 28 hours.
PRECAUTION
- When using Lenvima 10mg Hypertension reslted in 73% of Lenvatinib-treated patients in clinical trials (grade 3 hypertension was 44%); regulate blood pressure before to treatment; check blood pressure after 1 week, then q2weeks for the first 2 months.
- Lenvima 10mg causes Arterial thromboembolic events occurred in 5% of treated patients; stop drug following a thromboembolic event; forever stop treatment following an arterial thrombotic event.
- When using of Lenvima 10mg capsule will occurs Gastrointestinal perforation or fistula (2%); stop if patient experience a GI perforation or life-threatening fistula; permanently stop therapy in patients who have gastrointestinal perforation of any serious or Grade 3 or 4 fistula.
- While on treatment will reported Serious complications of poorly controlled hypertension.
- Discuss with the doctor if you are breastfeeding or plan to breastfeed. Avoid breastfeed during Lenvima 10mg treatment.
MISSED DOSE
- The dose of Lenvima 10mg is missed, and then takes the dose as you remember earlier. If time to next dose reaches, then swap the missed dose and continue the routine schedule. Avoid taking 2 doses at one time. Please consult your doctor for further clarification
STORAGE
- Store the drug at 2 ℃ – 8 ℃
- Avoid freezing or shake
- Protect from light
SIDE EFFECTS
Common side effects :
- Weight reduced
- Palmar-plantar erythrodysesthesia
- Protein urea
- Hand-foot syndrome
- Dyspnea
- Loss of appetite
- Difficulty speaking
- Heartburn
- Blood pressure increased
- Diarrhea
- Muscle aches
- Mouth sores
- Insomnia
- Dental & oral infection
- Taste changes
- Swelling
- Nose bleed
- Urinary tract infection
- Hair loss
- Taste changes
- Rash
CONTACT DETAILS
Phone : 9987711567
Email : applepharmaceutical@gmail.com
Email : info@myapplepharma.com